How To Count Electron Domains
8.6: Molecular Geometries
- Page ID
- 52692
Introduction
There is a three step approach to determining the geometry of a molecule.
- Determine the Lewis dot construction of the chemical compound.
- Determine the Electron geometry from the Lewis dot structure.
- Make up one's mind the molecular geometry.
It is very important from the onset that students understand the difference betwixt electronic geometry and molecular geometry. In calculating electronic geometry we utilise the Valence Shell Electron Pair Repulsion (VSEPR) model, which states that the lowest geometry for electronic orbitals around a positive nucleus is for the orbitals to exist as far away as possible. At present there are ii bones types of orbitals, bonding and nonbonding (lonely pair) orbitals. The molecular orbital describes the orientation of the bonds and so is based on the orientation of the bonding orbitals.
VSPER
VSEPR (Valence Beat Electron Repulsion Theory) is used to determine the geometry of the orbitals around a nucleus. In VSEPR all valence orbitals are considered to accept the aforementioned shape, in fact it may exist more appropriate to consider them as electron domains. That is, alone pairs, single bonds, double bonds and triple bonds are all treated equally an electron domain, and the VSPER (electronic) geometry is determined past the number of electron domains in the valence shell of an atom. In this form we will exist responsible for the geometry of that effect from the VSPER interactions of ii through vi orbitals.
Video \(\PageIndex{1}\) is a very curt video showing the five VSPER electronic geometries for atoms with between 2 and 6 electron domains, the steric number. In watching video \(\PageIndex{1}\) you should note how the geometry changes as the steric number changes and additional orbitals are successively added to the fundamental atom. The v geometries are summarized in table \(\PageIndex{1}\).
The 5 VSEPR geometries are summarized in table \(\PageIndex{1}\).
| # Electron Domains (Steric Number) | VSEPR Geometry | Bending Between Electron Domains | |
|---|---|---|---|
| 2 | linear | 180o |  |
| 3 | trigonal planar | 120o | 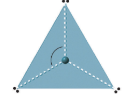 |
| 4 | tetrahedral | 109.5o |  |
| v | trigonal bipyramidal | 90o, 120o | 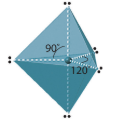 |
| 6 | octahedral | xco | 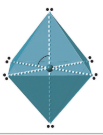 |
Molecular Geometry
Afterward calculating the electronic geometry from VESPR we can make up one's mind the molecular geometry based on the bonding orbitals. If there are no lone pairs and all orbitals are bonding, then the molecular geometry is the electronic geometry. Lonely pairs influence the molecular geometry, and then in this section we volition look at molecular geometries every bit subsets of electronic geometries.
Before proceeding, please watch the follow YouTube
Effigy \(\PageIndex{1}\) shows the various molecular geometries for the 5 VESPR electronic geometries with 2 to half dozen electron domains. When there are no lone pairs the molecular geometry is the electron (VESPR) geometry. When there are lone pairs, yous need to wait at the structure and recognize the names and bond angles. Note, this work ignores the trivial geometry of 2 atoms like HCl or H2, every bit they must be linear, simply when yous have three atoms, they tin be linear or bent.
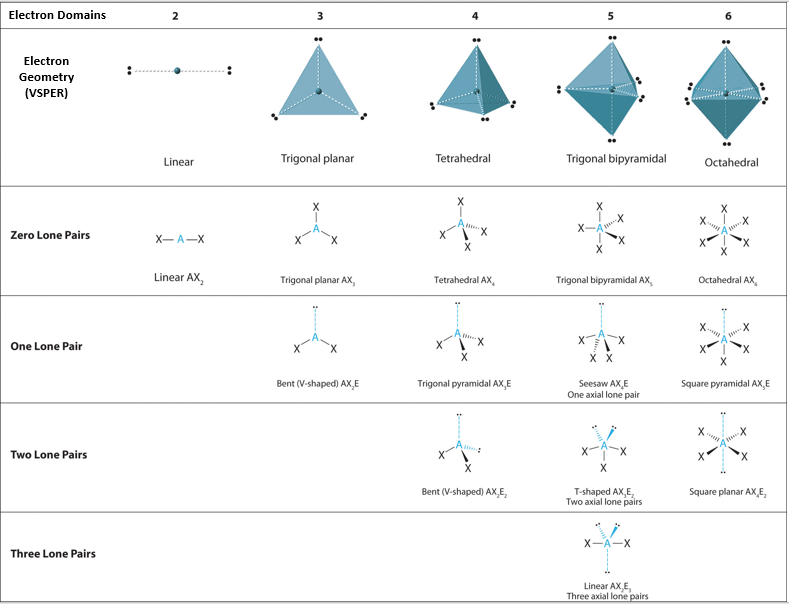
Figure \(\PageIndex{1}\): Overview of molecular geometries based on bonding orbitals of VSEPR electronic structures.
Ii Electron Domains
Three atoms event in two electron domains and the structure is linear. There are three common types of molecules that form these structures, molecules with two single bonds (BeH2), molecules with a two double bonds (CO2) and molecules with a single and triple bond (HCN).
| H-Be-H | O=C=O | \(H-C\equiv N\) |
| BeH2 | CO2 | HCN |
Annotation, Glucinium tin take less than an octet, while carbon can not. Likewise notation that the double bond in carbon dioxide and the triple bond in hydrogen cyanide are both treated as a single electron domain
Three Electron Domains
There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with \(\approx \)120° bond angle (one solitary pair) .
0 lone pairs
These are of the class AX3, where X represents an atom that is bonded to 3 other atoms, and for which at that place are no lone pairs.
1 lone pair
These are atoms of the form AX2E, where E represents a lone pair. Examples are So2 and O3. Notation, the lone pair takes up more space than the bonding pair, so the bond bending is less then the ideal 120o.

Four Electron Domains
All atoms with 4 electron domains have tetrahedral electronic geometry
0 Lone Pairs
These are of the form AX4 and the molecular geometry is the same as the electronic geometry
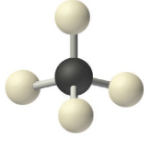
1 Lone Pair
These are of the form AX3E and take trigonal pyramidal molecular geometries. Note the bail angle is less than the ideal considering the lone pair take up more infinite

two Lone Pairs
These are of the class AX2E2 and have bent angles, which in the case of h2o are 104.fiveoC

These are of the form AX2Etwo and have bent angles, which in the instance of water are 104.voC
Notation
There are ii aptitude geometries based on trigonal planar electronic geometry with one solitary pair as exemplified by sulfur dioxide that has a bond angle a chip less than 120oC, and by tetrahedral electronic geometry with two lone pairs, every bit exemplified past h2o with 104.fiveoC bond angle.
V Electron Domains
All molecules with 5 electron domains have trigonal bipyramidial electronic geometry. The primal atom of these molecules must be in the tertiary or higher flow of the periodic tabular array.

In Figure \(\PageIndex{7}\) you note that the two axial positions are linear to each other and if we define this axis as the z axis of the cartesian coordinate organization, then the equatorial positions have a trigonal planar geometry in the xy airplane. Then the trigonal bipyramidal geometry is a superposition of linear and trigonal planar geometries. It is of import to note that the bond angle between equatorial and axial positions (90o) is dissimilar than between two equatorial positions (120o).
0 Solitary Pairs
As in the higher up cases, if in that location are no alone pairs, the electronic geometry is the molecular geometry.

1 Lone Pair
These are of the form AX4E and have a "Come across-Saw" geometry, which is too classified equally a distorted tetrahedron. Sulfur tetrafluoride ( SF4) has such a structure.
Annotation
The Solitary pair can have 2 positions, axial or equatorial. The solitary pair goes into the equatorial position because it takes up more space, and there is more than room in the equatorial positions. This tin can exist seen from Figure \(\PageIndex{7}\), where it is clear that the 90o bonds bring the atoms closer than the 120o bonds, and each axial position has 3 90o bond interactions while each equatorial has two (and two 120o) bond interactions. Equally the lone pairs take upwards more space, they move into the equatorial positions.

2 Lonely Pairs
These are of the class of AXthreeE2 have trigonal bipyramidal electronic geometry and "T-shaped" molecular geometry. Bromine triflouride (BrF3) is an example of a molecule with 5 electron domains and two alone pairs (Figure .

Figure \(\PageIndex{10}\): Lewis dot diagram of bromine trifluoride showing two lone pairs.
At that place are iii ways of distributing the lone pairs between the axial and equatorial positions and the solitary pairs always go along the equatorial positions considering these are the least bars. This can exist seen in Figure \(\PageIndex{11}\)

One time once more, the lone pairs go into the equatorial positions.
3 Lonely Pairs
These are of the form AX2East3 and and the trigonal bipyramidal electronic construction results in a linear molecular structure. Triiodide (I3 −) is an example of this geometry.

6 Electron Domains
Six electron domains course an octahedron, a polyhedron with 8 faces, but the electron pair geometry has linear orientations along the 3 Cartesian coordinate centrality. Therefore the octahedral represents 6 electron domains along the Cartesian axis (Figure \(\PageIndex{13}\)).
 Effigy \(\PageIndex{xiii}\): Half dozen domains has electron pairs oriented forth the 3 Cartesian coordinate axes
Effigy \(\PageIndex{xiii}\): Half dozen domains has electron pairs oriented forth the 3 Cartesian coordinate axes0 Alone Pairs
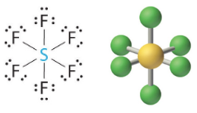
1 Lonely Pair

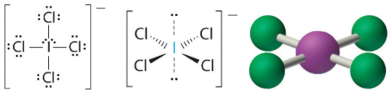
2 Lone Pairs
These structures are of the class AXfourE2and form square planar structures. Iodine tetrachloride ion (ICl4 −). T
Molecules with No Single Central Atom
The VSEPR model tin be used to predict the structure of somewhat more circuitous molecules with no single central atom by treating them as linked AX 1000 E n fragments. Nosotros volition demonstrate with methyl isocyanate (CH3–Due north=C=O), a volatile and highly toxic molecule that is used to produce the pesticide Sevin. In 1984, big quantities of Sevin were accidentally released in Bhopal, India, when water leaked into storage tanks. The resulting highly exothermic reaction caused a rapid increase in pressure that ruptured the tanks, releasing large amounts of methyl isocyanate that killed approximately 3800 people and wholly or partially disabled near l,000 others. In addition, there was significant damage to livestock and crops.
Nosotros can treat methyl isocyanate (CH3–Northward=C=O), equally linked AX k E north fragments beginning with the left carbon, followed past the nitrogen and so the second carbon
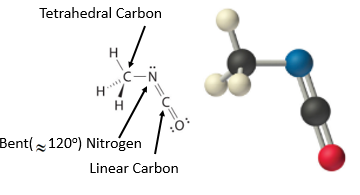
Figure \(\PageIndex{17}\): Geometric structure of methyl isocyanate (CHthree–N=C=O), note at that place is no rotation effectually the double bonds only the single CN bond can rotate.
Contributors and Attributions
Robert E. Belford (University of Arkansas Piddling Rock; Section of Chemistry). The breadth, depth and veracity of this piece of work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you lot have whatever concerns. This cloth has both original contributions, and content built upon prior contributions of the LibreTexts Customs and other resources, including but not limited to:
- Anonymous
- Modifications of cloth modified past Joshua Halpern, Scott Sinex and Scott Johnson
How To Count Electron Domains,
Source: https://chem.libretexts.org/Courses/University_of_Arkansas_Little_Rock/Chem_1402:_General_Chemistry_1_%28Belford%29/Text/8:_Bonding_and_Molecular_Structure/8.6:_Molecular_Geometries
Posted by: mclarenquity1983.blogspot.com


0 Response to "How To Count Electron Domains"
Post a Comment